Abstract
Introduction: Treatment combinations involving CD38 targeted monoclonal antibodies have significantly prolonged the median duration and depth of response in myeloma (MM), reflected in minimal residual disease-negativity (MRD-) rates of over 70% in newly diagnosed patients (Landgren et al. JAMA Onc 2021). Key to improving our understanding of treatment failures is the combined use of single cell analysis of the microenvironment with genome wide assessment of tumor genetics to decipher the mechanisms of disease resistance.
Methods: We isolated malignant plasma cells from bone marrow (BM) samples using CD138+magnetic or flow (CD38, CD138, and CD45) sorting from 60 newly diagnosed MM patients treated with KRd with daratumumab (D-KRd n=46; NCT03290950) and without daratumumab combination therapy (KRd, n=14; NCT01402284). Fifty-five baseline samples underwent whole genome sequencing (WGS), median coverage of 80x using somatic DNA as a normal comparator. The BM cellular content of 22 patients (44 samples-5 failed) treated with D-KRd (10 MRD+ and 12 MRD-) underwent 5'single cell RNA-sequencing with an additional capture of the TCR and surface protein markers (CITEseq) to interrogate the single cell composition of the immune microenvironment at baseline (T1, n=20) and at the end of induction (T2, n=19). Paired (T1/T2) single-cell data were obtained in 17 patients and paired WGS and single cell data (T1) were available in 15 patients. MRD-, sustained MRD- (defined as two MRD- results, the first at the end of the induction (T2) and the subsequent at the first year of follow-up (T3)) and progression/loss of MRD- were used as clinical endpoints for this study.
Results: After a median follow up of 29 months, 36 (54%) patients achieved MRD-; 34 (51%) had sustained MRD- >1 year after completion of combination therapy. Overall, 10 (15%) patients had clinical progression and two conversions from MRD- to MRD+. A comprehensive catalogue of MM-genomic events associated with these three clinical endpoints was defined. Deletion (del) 13, biallelic loss CYLD, del XBP1, del 20q13.12 (CD40), and 8q gains were associated with MRD+ and failure to achieve sustained MRD-. Presence of del RPL5 and multiple chromothripsis events significantly correlated with early progression and loss of MRD-. Interestingly, structural variants (SV) involving IKFZ3 were seen in all three negative clinical endpoints (p<0.05) and also associated with early progression in the CoMMpass data set (p=0.01, n=12). Trisomy 21 emerged as a favorable subset (11/13 sustained MRD- cases p=0.02) and was also seen in CoMMpass (p=0.003). Overall, these data highlight potential novel genomic drivers associated with resistance to D-KRd.
We interrogated the BM microenvironment at baseline and correlated its composition with the tumor genomic architecture. Across 15 evaluable patients, del XBP1 were associated with fewer memory B-cells (p=0.03), naïve B-cell (p=0.01) and dendritic cells (p=0.03) compared to the wild type. Also, low dendritic cell at baseline cases were observed in patients with del 20q13.12 (CD40) (p=0.03). Interestingly, low level of plasmacytoid dendritic cells at baseline was associated with failure to achieve MRD- and sustained MRD-. Patients with 6p24 amplification showed a reduced number of CD8 effectors 1 and 2 (p<0.05). Overall, these data suggest that distinct genomic lesions are associated with distinct immune-microenvironment composition.
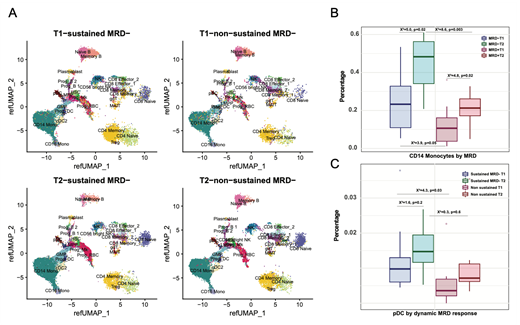
When comparing baseline (T1) and end of induction (T2), significant differences were seen between sustained MRD+ and MRD-. We identified significantly depleted NK, and naïve and memory B-cell after D-KRd MRD- patients had significantly more CD14+ monocytes both at T1 and T2 than their MRD+ counterparts (Fig. 1). Differential expression suggests that inflammatory response genes including IL1B are upregulated in the absence of sustained MRD- whereas genes implicated in IL2, IL6, and IFNα response as well as adipocyte differentiation are associated with sustained MRD response.
Conclusion: We show, for the first time, evidence of complex interplay between MM tumor genetics and the microenvironment in the context of D-KRd treated patients. Our results highlight the importance of genomic-based mechanisms in the persistence of disease (IKZF3, XBP1) as well as heterogeneity in the composition of the BM microenvironment, with the monocytes pointing towards the importance of inflammation.
Maura: OncLive: Honoraria; Medscape: Consultancy, Honoraria. Hassoun: Celgene, Takeda, Janssen: Research Funding. Mailankody: Jansen Oncology: Research Funding; Allogene Therapeutics: Research Funding; Bristol Myers Squibb/Juno: Research Funding; Legend Biotech: Consultancy; Takeda Oncology: Research Funding; Fate Therapeutics: Research Funding; Physician Education Resource: Honoraria; Plexus Communications: Honoraria; Evicore: Consultancy. Hultcrantz: Intellisphere LLC: Consultancy; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding; Curio Science LLC: Consultancy; Amgen: Research Funding; Daiichi Sankyo: Research Funding. Scordo: Omeros Corporation: Consultancy; i3 Health: Other: Speaker; Kite - A Gilead Company: Membership on an entity's Board of Directors or advisory committees; Angiocrine Bioscience: Consultancy, Research Funding; McKinsey & Company: Consultancy. Kazandjian: Arcellx: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Shah: Janssen Pharmaceutica: Research Funding; Amgen: Research Funding. Landau: Takeda, Janssen, Caelum Biosciences, Celgene, Pfizer, Genzyme: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Genzyme: Honoraria. Giralt: SANOFI: Membership on an entity's Board of Directors or advisory committees; PFIZER: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; JAZZ: Membership on an entity's Board of Directors or advisory committees; JANSENN: Membership on an entity's Board of Directors or advisory committees; AMGEN: Membership on an entity's Board of Directors or advisory committees; Actinnum: Membership on an entity's Board of Directors or advisory committees; CELGENE: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Dogan: Physicians' Education Resource: Honoraria; Seattle Genetics: Consultancy; EUSA Pharma: Consultancy; Roche: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Peer View: Honoraria. Lesokhin: Behringer Ingelheim: Honoraria; pfizer: Consultancy, Research Funding; Janssen: Honoraria, Research Funding; Iteos: Consultancy; Genetech: Research Funding; bristol myers squibb: Research Funding; Trillium Therapeutics: Consultancy; Serametrix, Inc: Patents & Royalties. Davies: Janssen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Korde: Amgen: Research Funding; Medimmune: Membership on an entity's Board of Directors or advisory committees. Morgan: BMS: Membership on an entity's Board of Directors or advisory committees; Jansen: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees. Landgren: Janssen: Other: IDMC; Celgene: Research Funding; Amgen: Honoraria; Janssen: Research Funding; Janssen: Honoraria; Amgen: Research Funding; Takeda: Other: IDMC; GSK: Honoraria.